The product world is often mesmerized by the rapid innovation in the software field. But, Pradeep Vamana, co-founder of Prodancy, is a staunch evangelist for the tangible, the meticulously engineered, and the beautifully designed hardware product.
With a rich 25-year career spanning aerospace engineering, global procurement strategies at giants like Airbus, and a hard-earned aerospace MBA from France, Pradeep Vamana, alongside his illustrious partner Venkatesh Parthasarathy (Venky), is championing a movement to put Indian-designed and manufactured hardware on the global map.
Prodancy, at its heart, is a tale of two distinct yet deeply intertwined ambitions. It’s a product design consultancy powerhouse, specializing in the often-underestimated realm of hardware. Simultaneously, it’s a proud parent of its own line of innovative products, particularly medical devices, born from deep market understanding and rigorous design principles.
With a dynamic team of 25, Prodancy is rapidly carving a niche as one of India’s leading product design firms. In late 2024, they successfully raised ₹2.14 crore in a funding round co-led by Campus Angels Network and Keiretsu Forum Chennai, which also saw participation from existing investors C-CAMP and other angel investors. Here’s their story.
A 25-Year Partnership and a Pandemic Pivot
Pradeep and Venky’s journey began in 1996, as college mates undertaking a specialized diploma in tool and die making, a discipline focused on the intricate world of plastic injection mold design. This foundational understanding of manufacturing processes (plastics, sheet metal, die-casting, etc.,) became the bedrock of their future endeavors.
While Pradeep veered into casting, forging simulation, and eventually a distinguished career in aerospace, working with industry titans like Airbus and Pratt & Whitney across the US and Europe, Venky delved deep into metal injection molding and, critically, industrial design.
"We've been friends for the last 30 years," Pradeep shares. "I was proposing to start a company together for almost 20-25 years now. And finally, we started the company in 2020 right when the COVID-19 pandemic hit the world."
The pandemic, however, became an unexpected catalyst. “Because of COVID, we went into medical devices. Like everybody, we initially designed for ventilators, but realized it was a long project. It’s not going to happen in three months.”
But a more personal need arose, which gave them the jumpstart. Venky’s sister, a dentist, required a respiratory protection device. This spark of necessity fanned into what is now one of Prodancy’s flagship products – the Vizbl surgical helmet.
Protecting the Protectors - An Unmet Need

"During COVID, respiratory protection devices for healthcare became a very important aspect," Pradeep explains. "Even today, for that matter, there is no good device that's available in the market in terms of respiratory protection."
"It's very, very heavy...about 1.3 kilograms. It is also very expensive at almost three lakhs per unit. Wearing that and doing surgery is painful. Surgeons don't like it."
The result? A surgical helmet that, since its launch in April 2023, is now used by over 350 surgeons across India, who have collectively performed approximately 17,500 surgeries. It’s a testament to Prodancy’s ability to identify a critical pain point and engineer a targeted solution.
Esteemed institutions like Narayana Health, Apollo Hospitals, Fortis, Max Healthcare, Lilavati Hospital, and Kokilaben Hospital are now among their clientele. This product, Pradeep proudly states, has allowed them to acquire a “significant market share from competition” in just two years.
Alongside the helmet, Prodancy has also developed products such as a cryo-compression brace for post-surgery and injury recovery, as well as a specialized wrist support for Carpal Tunnel Syndrome, now available on Amazon.
These products are Prodancy’s own IP, distinct from the impressive portfolio of consulting projects their team, particularly Venky, has designed. Over the years, he has led the design and delivery of everything from Commonwealth Games and Formula One trophies to advanced medical diagnostic tools and consumer electronics for renowned brands like Butler and Morphy Richards.
The Prodancy Playbook: Function, Aesthetics, and User-Centricity
For product leaders and founders reading ProdWrks, Prodancy’s approach to product development (from idea to manufacturing) offers a masterclass in transforming an idea into a market-ready reality, especially in the demanding hardware space.
Pradeep outlines a journey that is both systematic and deeply empathetic.
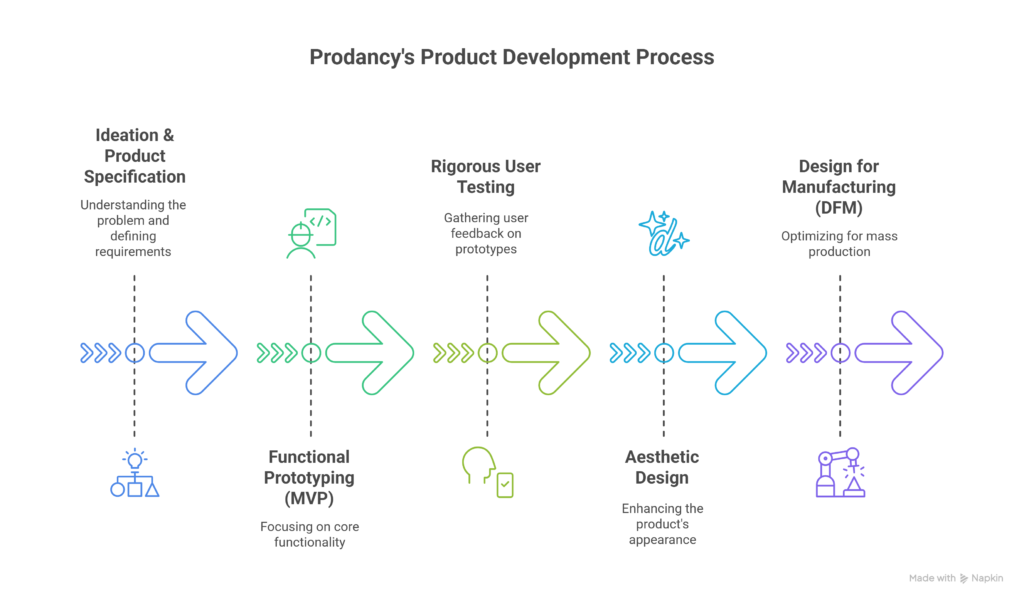
1. Ideation & Product Specification:
It begins with understanding the problem.
"Most product founders don't get this right," Pradeep cautions. "They come with an idea, but it's very vague, and they jump straight away into product development. Through experience, we don't go into development until we put a solid product requirement document together."
2. Functional Prototyping (The MVP):
"It's like an onion," Pradeep describes. "We start with the product core, and then start to build the layers around it."
The focus here is solely on making the core functionality work flawlessly. “It doesn’t need to look good, but it must function well.” This is where many hardware startups falter, mixing aesthetic concerns too early.
Venky at Prodancy insists on solving function first.
3. Rigorous User Testing with Crude Prototypes:
"At this stage, we recommend that founders take their prototype to users. When you put a functional prototype in the hands of a user, they will tell you exactly what they like and what they don't. Most people sit in their armchairs and imagine that the world is different, which is usually not the case."
"In a software product, you would use a heatmap, for example. Whereas in a hardware product, you make video of the user using your product," Pradeep explains. More crucially, "physically, you should be there in front of the customer, deeply observe them as much as possible and speak their language."
This commitment led Pradeep himself, despite his aerospace background, to observe nearly 100 knee surgeries. “To the extent that we can have a decent conversation with surgeons about knee surgery. To speak the language of your user is very, very critical.”
Finding the right users is paramount. “Qualifying an ideal user while doing testing is critical. Pick the user who’s technically sound, who’s mostly critical of the product, but very structured in their feedback.” He advocates for the “Five Whys” technique to delve deep into the rationale behind feedback, filtering out mere opinions.
4. Aesthetic Design:
Only after the function is nailed and user feedback is incorporated does the focus shift to making the product beautiful. “This is where you know exactly who your customer is. You create a brand, you create a mood board, you create a design language, then create an aesthetic design.”
This stage involves 3D printing, painting, and secondary operations to create prototypes that are virtually indistinguishable from finished products for a layperson.
5. Design for Manufacturing (DFM) and Testing:
"We are experts in materials. We understand manufacturing processes innately - the fits and tolerances, the aesthetics of it, etc.," Venky asserts.
The Iteration Game: Patience and Precision in a High-Stakes Field
"It can even take 50 to 60 prototypes if you're trying to build a product for a problem that's unsolved," Pradeep admits, a figure that might seem daunting to early-stage founders. "It's a bit disappointing, but that's the reality of it."
"Product design is a series of experiments. The more precise you are in terms of the goal or objective of an experiment, the more precise you will be in terms of what you're going to put money into."
He credits his partner Venky with instilling a crucial discipline: “Until the function is solved, he will not go into aesthetics, no matter what. That precise, dedicated focus, is very important.”
This helps avoid getting sidetracked by aesthetic feedback when functional issues are still unresolved, a common trap for hardware-first startups.
Navigating the "Brutal" MedTech Landscape in India
"Medical device is brutal. Every product is brutal, but product development in medical devices and launching them to the mass market, relatively, is more brutal."
The reasons are threefold:
- Niche Markets & Entrenched Competition: Medical devices often serve highly specific niches. “If the market is large enough, the problem is large enough, then there are already a lot of players there who have covered the market and patented their products and designs. So you have to break through the patent cycle as well.”
- The Gauntlet of Validation: “Proving it (your product) is harder, because you’re talking about clinical trials, finding the right people to do clinical trials, and the trials working out. And finally, you have regulatory hurdles.”
- The Sales Quagmire: “Selling to healthcare providers is a nightmare. Typical hospitals have 90-days payment cycle. The moment you are an Indian company and showcase an Indian medical device, they’ll be like, ‘Oh, Indian product. I’ll tell you what the pricing should be.’ Whereas a foreign product will be 3x the price, and they’re happy to pay.”
Despite these formidable challenges, Pradeep sees immense value in the Indian market.
"What India can teach, I think, no global market can teach you. If you sell in India, you can sell anywhere in the world. Indian market may be brutal, but it is extremely valuable."
He believes that if a product succeeds in India, its profitability will only multiply in international markets. This conviction led him to move back to India from the US in 2022 to build Prodancy.
“What I could get in the last three years of experience, if I were to do that same thing in the US, I think it is equal to at least nine years of experience in the US.”
Prodancy’s go-to-market strategy for their own products bypasses traditional distributors.
“We directly work with the surgeons. We enable them to experience our product, and then we talk about commercials if they’re interested. In our case, we have a 95% conversion ratio.”
This direct engagement, especially in the early stages by targeting experts who have just started their own hospitals (thus cutting through corporate red tape), provides rapid, unfiltered feedback.
The Funding Blindspot in India’s Hardware Dream
For product leaders and founders in the hardware space, Pradeep’s perspective on the investment landscape, particularly in India, will resonate deeply. He paints a picture of a venture capital community often misaligned with the realities of building tangible products and not having the in-house expertise to understand the hardware product play.
He contrasts the Indian scenario with the specialized VCs in mature markets like the US and Europe where investors might focus solely on (for instance) diabetic products or surgical robotics.
"In India, most investors would say that, 'Hey, I'm stage agnostic and industry agnostic. I can invest in any startup.' Basically, they're saying ‘I'm Jack of all, Master of None.’"
"All they want to know is, okay, what is the risk of me putting this money? And when do I get my 3x? And we get ridiculous questions like, 'Okay, when can this company become $100 million company?'"
The common refrain, “market chota hai” (the market is small), frustrates Pradeep, who points out that even a $2 billion global market for a niche medical device is substantial.
He points to the massive capital burn in sectors like D2C and EdTech, often with questionable long-term value creation, compared to the capital efficiency and IP generation potential of hardware.
"If 300 crores were invested in medical device companies, the amount of medical devices that we can create is beautiful, and you can create profitable businesses. We can reduce the current 80% medical device imports. Fundamentally, if you're not building a profitable business, what is the purpose of being an entrepreneur?"
Pradeep believes that the investor’s focus on revenue growth at the expense of profitability is a flawed model. He believes that deep-tech hardware companies like Agnikul, Mindgrove, Plovinc (maker of the Play Shifu), Ather Energy, and Ultraviolette Automotive are the true product champions India should celebrate.
He proposes a solution for fostering a thriving medtech hardware ecosystem.
"In India, medical devices are not VC fundable. It is not government fundable. So the government, in my view, has to create a Venture Lab setup where you have a common sales team for all medical device companies. Your regulatory teams and your manufacturing teams can be common, whereas your research R&D team can be different."
Embracing AI Co-Pilot and Envisioning the Future
This technological acceleration, he predicts, will lead to "products that can have quick iterations in terms of design configurations and time to market. This will promote more low-volume, but, high-value production with shorter iteration cycles.”
"We have to create a framework around product development to combine our capabilities as entrepreneurs, for the nation. We have to accelerate our product development lifecycle. If we can focus on that, we save a lot of money and save a lot of equity. At Prodancy, we will be happy to support any hardware founder in their product development."



